
Isomorphic Labs, an independent business unit of Alphabet, has announced a strategic research collaborations with pharmaceutical giants Eli Lilly and Novartis. The deals focus on applying Isomorphic's AI technologies, including the latest iteration of DeepMind's revolutionary AlphaFold protein folding software, to identify and design small molecule therapies against multiple disease targets.
Under the terms of the Lilly agreement, Isomorphic receives $45 million upfront and is eligible for up to $1.7 billion in performance-based milestone payments, excluding royalties, to discover treatments across several undisclosed therapeutic areas.
Google DeepMind Co-founder & CEO Demis Hassabis, and who is also in charge of Isomorphic Labs, expressed enthusiasm about applying their advanced AI and massive computing power to Lilly's development programs.
The Novartis collaboration also centers on small molecule discovery against three targets, with Isomorphic receiving $37.5 million upfront plus research cost funding and eligibility for $1.2 billion in milestones, again excluding royalties.
Fiona Marshall, President of Biomedical Research at Novartis, recognized the transformative potential of AI technologies like AlphaFold in drug discovery. This collaboration aims to combine strengths in AI, data science, medicinal chemistry, and deep disease area expertise to explore new frontiers in AI-driven drug discovery.
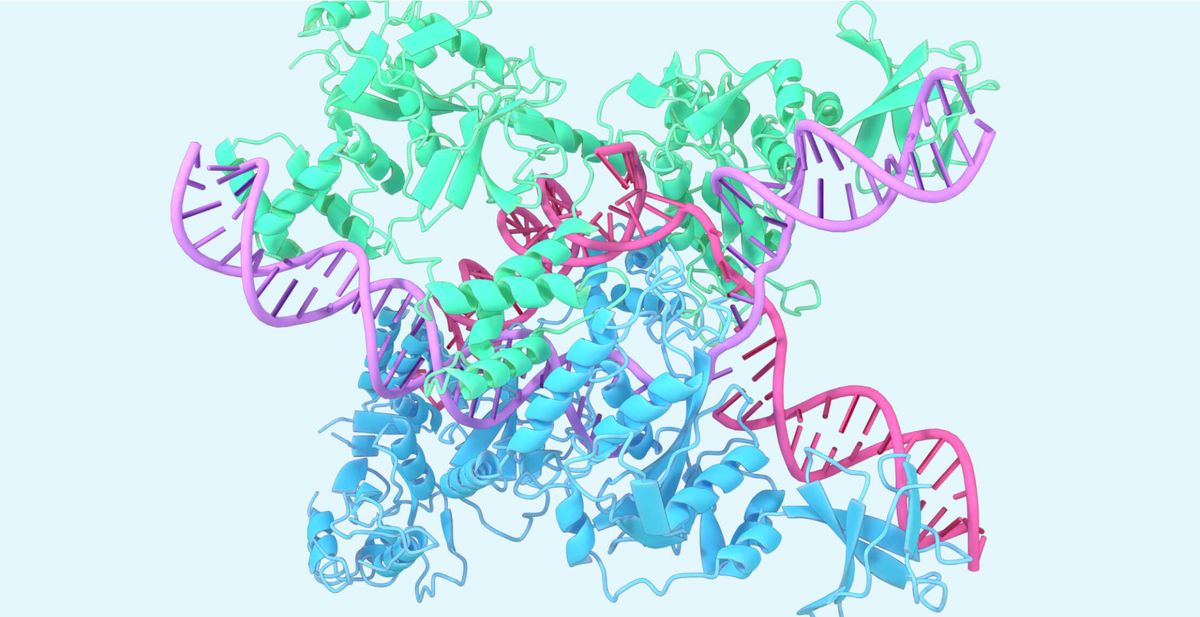
Central to Isomorphic's tech platform is its next generation AlphaFold system, which builds on DeepMind's breakthroughs in protein structure prediction. The new model shows improved performance in predicting protein-ligand structures, crucial for drug discovery. It surpasses traditional docking methods, allowing predictions for novel proteins without needing a rigid reference structure. This capability is vital for designing new therapeutic molecules and understanding flexible interactions between proteins, nucleic acids, and other molecules.
The model has been instrumental in accurately predicting structures of clinically relevant molecules, such as PORCN (an anti-cancer molecule), KRAS (a cancer target), and PI5P4Kγ (a kinase with implications in cancer and immunological disorders). These advancements illustrate the model's potential to revolutionize therapeutic drug design and deepen our understanding of fundamental biology, including structures like CasLambda in the CRISPR system.
Integrating those predictions with other novel Isomorphic AI models provides atomic-level understanding of therapeutic targets and mechanisms of action, enabling rational design of potential treatments.
With a combined value of about $3B, the high-profile deals underscore the immense faith that industry leaders have in AI-driven drug discovery as the future of medicine R&D. While the technology has many limitations, these collaborations represent a ringing endorsement of Isomorphic's generative AlphaFold platform and novel approach. Much as AlphaFold fundamentally advanced protein science, its next generation may spark an AI-powered revolution in drug development.


